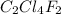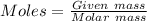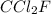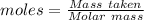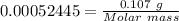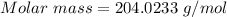Chemistry, 27.09.2019 02:30 tateandvioletAHS14AY
Analysis of a gaseous chlorofluorocarbon, cclxfy, shows that it contains 11.79% c and 69.57% cl. in another experiment, you find that 0.107 g of the compound fills a 458-ml flask at 25 °c with a pressure of 21.3 mm hg. what is the molecular formula of the compound?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, SmolBeanPotato
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 22.06.2019 07:30, zamirareece17
1. list three scientific reasons cockroaches may fly.
Answers: 1

Chemistry, 22.06.2019 07:30, avisconti571
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2
You know the right answer?
Analysis of a gaseous chlorofluorocarbon, cclxfy, shows that it contains 11.79% c and 69.57% cl. in...
Questions in other subjects:





Law, 08.03.2021 07:30



Mathematics, 08.03.2021 07:30


Mathematics, 08.03.2021 07:30