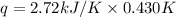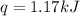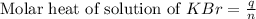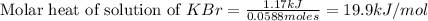Chemistry, 27.09.2019 02:30 bracefacer42
When a 7.00 g7.00 g sample of kbrkbr is dissolved in water in a calorimeter that has a total heat capacity of 2.72 kj⋅k−1,2.72 kj⋅k−1, the temperature decreases by 0.430 k.0.430 k. calculate the molar heat of solution of kbr.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, devontemiles8868
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1

Chemistry, 22.06.2019 15:00, emmalie52
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
When a 7.00 g7.00 g sample of kbrkbr is dissolved in water in a calorimeter that has a total heat ca...
Questions in other subjects:




Social Studies, 06.01.2020 16:31

Mathematics, 06.01.2020 16:31

Mathematics, 06.01.2020 16:31

Mathematics, 06.01.2020 16:31

Mathematics, 06.01.2020 16:31

Mathematics, 06.01.2020 16:31

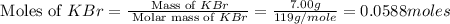
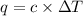
 = heat capacity =
= heat capacity = 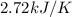
 = change in temperature = 0.430 K
= change in temperature = 0.430 K