
Which of the following statements concerning the attraction of ions to polar molecules is/are correct? 1. the force of attraction between an ion and a polar molecule is inversely proportional to the square of the distance between the center of the ion and the oppositely charged pole of the dipole. 2. the higher the ion charge, the stronger the attraction between the ion and a polar molecule. 3. the greater the magnitude of the dipole, the greater the attraction between the ion and a polar molecule.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:50, hadwell34
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 23.06.2019 03:00, duplessistoccara
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 2

Chemistry, 23.06.2019 03:00, rhianna18
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 1

Chemistry, 23.06.2019 03:00, yoongislaugh
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3
You know the right answer?
Which of the following statements concerning the attraction of ions to polar molecules is/are correc...
Questions in other subjects:





Mathematics, 25.06.2019 12:00

Chemistry, 25.06.2019 12:00



English, 25.06.2019 12:00

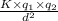
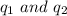 are the charges on the dipole and ion respectively.
are the charges on the dipole and ion respectively.


