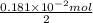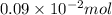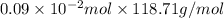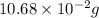Chemistry, 26.09.2019 22:20 dramaqueenactr2040
Find the percent by mass of tin in the original sample, assuming that it contains no other reducing agents. a sample of impure tin of mass 0.528 g is dissolved in strong acid to give a solution of sn2+. the solution is then titrated with a 0.0448 m solution of no3−, which is reduced to no(g). the equivalence point is reached upon the addition of 4.03×10−2 l of the no3− solution.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 23:00, fastpitchhailey1354
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1

Chemistry, 22.06.2019 14:00, coylenoah0
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2
You know the right answer?
Find the percent by mass of tin in the original sample, assuming that it contains no other reducing...
Questions in other subjects:


English, 11.06.2021 20:30




English, 11.06.2021 20:30

Mathematics, 11.06.2021 20:30



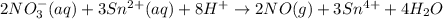
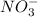 consumed will be calculated as follows.
consumed will be calculated as follows.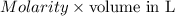
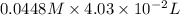
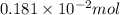
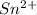 .
.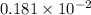 moles of
moles of