Chemistry, 26.09.2019 21:30 dayanaraa61
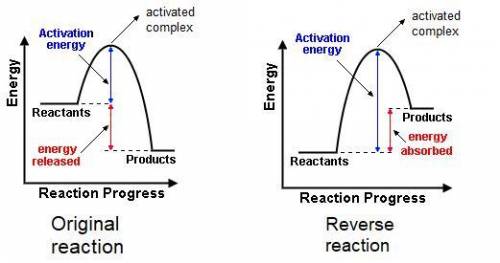
The gas phase reaction: cl(g) + hbr(g) →hcl(g) + br(g)has an overall enthalpy change of −66 kj. the activation energy for the reaction is 7 kj. a) draw the potential energy curve for the reaction and label ea, ∆e, and the activated complex or transition state. b) draw the potential curve for the reverse reaction. what is ea? what is ∆e?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, zayam1626
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2


Chemistry, 22.06.2019 16:00, anferneebcoleman
How many moles of oxygen react with 12 moles of aluminum
Answers: 1

Chemistry, 22.06.2019 22:10, steven0448
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
The gas phase reaction: cl(g) + hbr(g) →hcl(g) + br(g)has an overall enthalpy change of −66 kj. the...
Questions in other subjects:

Mathematics, 18.09.2021 05:00

Mathematics, 18.09.2021 05:00

Mathematics, 18.09.2021 05:00


Mathematics, 18.09.2021 05:00