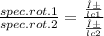An aqueous solution containing 10 g of an optically pure compound was diluted to 500 ml with water and was found to have a specific rotation of −129°. if this solution were mixed with 500 ml of a solution containing 7 g of a racemic mixture of the compound, what would the specific rotation of the resulting mixture of the compound? what would be its optical purity?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:30, kiaramccurty
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 19:00, nayashuntel
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
You know the right answer?
An aqueous solution containing 10 g of an optically pure compound was diluted to 500 ml with water a...
Questions in other subjects:






Mathematics, 29.06.2021 23:40

Mathematics, 29.06.2021 23:40

History, 29.06.2021 23:40


Mathematics, 29.06.2021 23:40