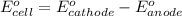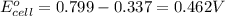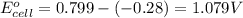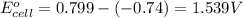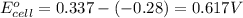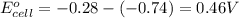Chemistry, 26.09.2019 16:30 savage5447
The standard reduction potentials of the following half-reactions are given in appendix e in the textbook:
ag+(aq)+e−→ag(s)= .799
cu2+(aq)+2e−→cu(s)= .337
ni2+(aq)+2e−→ni(s)= -.28
cr3+(aq)+3e−→cr(s). = -.74
1. determine which combination of these half-cell reactions leads to the cell reaction with the largest positive cell emf.
1st and 2nd,
1st and 3rd,
1st and 4th,
2nd and 3rd,
3rd and 4th.
it isn't the first or last one because i have gotten it wrong twice.
2. calculate the value of this emf.
3. then determine which combination is the smallest and calculate the emf.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, Tyrant4life
Amass of 100.0 g of solute is dissolved in water so that 850. ml of a 0.7500 m solution has been prepared. what is the molar mass of the solute?
Answers: 2



Chemistry, 22.06.2019 19:30, amandamiro05
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2
You know the right answer?
The standard reduction potentials of the following half-reactions are given in appendix e in the tex...
Questions in other subjects:

Mathematics, 25.05.2021 02:30

Mathematics, 25.05.2021 02:30






Computers and Technology, 25.05.2021 02:40


Mathematics, 25.05.2021 02:40

 potential will always get reduced and will undergo reduction reaction.
potential will always get reduced and will undergo reduction reaction.
 of the reaction, we use the equation:
of the reaction, we use the equation: