
Chemistry, 26.09.2019 16:30 costel8532
An insulated thermos contains 150 g of water at 87.3 ˚c. you put in a 10.2 g ice cube at 0.00 ˚c to form a system of ice + original water. the specific heat of liquid water is 4190 j/kg•k; and the heat of fusion of water is 333 kj/kg. what is the net entropy change of the system from then until the system reaches the final (equilibrium) temperature?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 10:30, cjtambasco
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 3.75 mol fe and 8.70 mol nio(oh) react?
Answers: 1

You know the right answer?
An insulated thermos contains 150 g of water at 87.3 ˚c. you put in a 10.2 g ice cube at 0.00 ˚c to...
Questions in other subjects:

Advanced Placement (AP), 19.10.2019 02:30

Physics, 19.10.2019 02:30



History, 19.10.2019 02:30

Chemistry, 19.10.2019 02:30

History, 19.10.2019 02:30



Mathematics, 19.10.2019 02:30

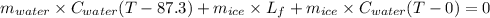
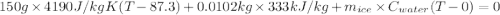
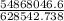
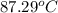

![\frac{0.0102 [\frac{333000}{273.15} + 4190 \times ln (\frac{360.44}{273.15})]](/tpl/images/0265/1324/75975.png)
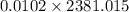
 ) =
) = 
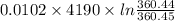
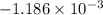 J/K
J/K
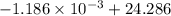 J/K
J/K

