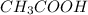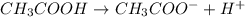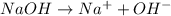Which statement best describes the conductivity of weak electrolytes, such as weak acids and bases?
a) weak electrolytes do not dissolve in water and can not conduct electricity.
b) weak electrolytes dissolve and partially dissociate in water providing charged ions to conduct electricity.
c) weak electrolytes dissolve and completely dissociate in water providing charged ions to conduct electricity.
d) weak electrolytes dissolve and does not dissociate in water providing no charged ions to conduct electricity.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:20, missayers172
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3


You know the right answer?
Which statement best describes the conductivity of weak electrolytes, such as weak acids and bases?...
Questions in other subjects:

Mathematics, 30.10.2020 01:00





Mathematics, 30.10.2020 01:00


Mathematics, 30.10.2020 01:00


Mathematics, 30.10.2020 01:00