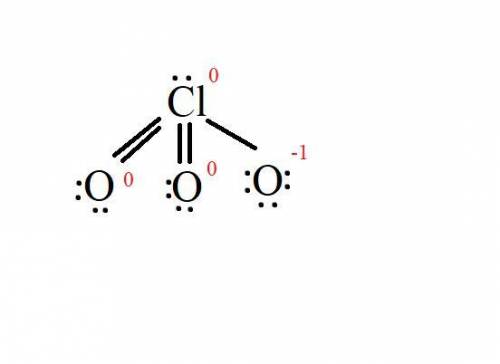
Based on formal charges, draw the most preferred lewis structure for the chlorate ion, clo3−. to add lone pairs, click the button before clicking on the molecule. to add bonds connect atoms with a line . to add formal charges, click the or button before clicking on the molecule. draw the molecule by placing atoms on the grid and connecting them with bonds. include lone pairs of electrons and formal charges.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, esnyderquintero
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 11:50, bellojamilet410
What substance has a mass of 9.5g and volume of 2.1cm^3
Answers: 2

Chemistry, 22.06.2019 18:30, tanviknawale
Which sample at stp has the same number of atoms as 18 liters of ne at stp
Answers: 1
You know the right answer?
Based on formal charges, draw the most preferred lewis structure for the chlorate ion, clo3−. to add...
Questions in other subjects:


Mathematics, 06.01.2021 02:20


Biology, 06.01.2021 02:20


Mathematics, 06.01.2021 02:20

Chemistry, 06.01.2021 02:20

Mathematics, 06.01.2021 02:20


Mathematics, 06.01.2021 02:20