
Chemistry, 25.09.2019 02:01 hlgerardip4wbhx
Determine the concentrations of mgcl2, mg2+, and cl− in a solution prepared by dissolving 2.52 × 10−4 g mgcl2 in 2.00 l of water. express all three concentrations in molarity. additionally, express the concentrations of the ionic species in parts per million (ppm).

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, bobbycisar1205
How does a hydroelectric power plant converts energy into energy.
Answers: 1



Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
You know the right answer?
Determine the concentrations of mgcl2, mg2+, and cl− in a solution prepared by dissolving 2.52 × 10−...
Questions in other subjects:

English, 22.04.2020 20:02





Biology, 22.04.2020 20:02




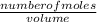

 = 0.000252moles
= 0.000252moles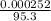 = 2.64 x 10⁻⁶moldm⁻³
= 2.64 x 10⁻⁶moldm⁻³


