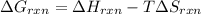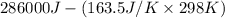Chemistry, 25.09.2019 02:20 destinyhammons12345
Determine the electrical work required to produce one mole of hydrogen in the electrolysis of liquid water at 298°k and 1 atm. the chemical reaction is h2001) h2(g) + 0.502(g) data (at 298°k and 1 atm): ah = 286 kj for this reaction, suzo = 70 jk, sh2 = 131 jik, and soz = 205 j/ºk.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, giusto1894
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 11:50, tajanaewilliams77
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 15:00, tcapele252
‘which reaction would most likely require the use of an inert electrode?
Answers: 1
You know the right answer?
Determine the electrical work required to produce one mole of hydrogen in the electrolysis of liquid...
Questions in other subjects:


English, 20.11.2021 16:40


Chemistry, 20.11.2021 17:10

Mathematics, 20.11.2021 17:10

Biology, 20.11.2021 17:10

Biology, 20.11.2021 17:20

Mathematics, 20.11.2021 17:20

Mathematics, 20.11.2021 17:20

English, 20.11.2021 17:30

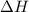 = 286 kJ =
= 286 kJ = 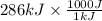
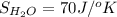 ,
, 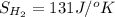
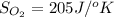
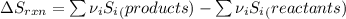
![[(\frac{1}{2} \times S_{O_{2}}) - (1 \times S_{H_{2}})] - [1 \times S_{H_{2}O}]](/tpl/images/0260/0439/5a90c.png)
![[(\frac{1}{2} \times 205) + (1 \times 131)] - [(1 \times 70)]](/tpl/images/0260/0439/31e9d.png)