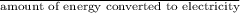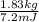Calculate the amount of co2 (in kg) released when 1 kg coal is burnt. assume that carbon content of the coal is 50% by mass. 4. calculate the co2 production in kg/mj if a coal fired power plant (efficiency - 30%) is used to produce electricity. assume energy density of coal - 24 mj/kg and assume the coal is

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, HHHHHHHHHMMMMMMMMM
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 16:00, ghadeeraljelawy
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 22.06.2019 21:30, emmalucilleblaha1995
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
Calculate the amount of co2 (in kg) released when 1 kg coal is burnt. assume that carbon content of...
Questions in other subjects:


English, 22.10.2021 21:10

History, 22.10.2021 21:10







English, 22.10.2021 21:10

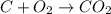
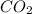 = 44 kg/kmol
= 44 kg/kmol
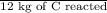
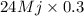 = 7.2 Mj
= 7.2 Mj