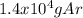Argon is contained in a piston-cylinder arrangement at a temperature of 100°c and a pressure of 200 kpa. an isothermal process takes the argon to a pressure of 50 kpa while 1,500 kj of heat are transferred to the arrangement. calculate: a) the amount of work produced during the process, in kj. b) the amount of mass in the piston-cylinder arrangement.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:00, Britny2386
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1

Chemistry, 22.06.2019 03:30, jabper5522
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1


Chemistry, 22.06.2019 16:00, anferneebcoleman
How many moles of oxygen react with 12 moles of aluminum
Answers: 1
You know the right answer?
Argon is contained in a piston-cylinder arrangement at a temperature of 100°c and a pressure of 200...
Questions in other subjects:


Mathematics, 09.10.2021 20:30





Mathematics, 09.10.2021 20:30

Mathematics, 09.10.2021 20:30