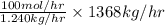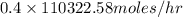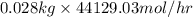Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, kkelley9223
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 09:00, angelrenee2000
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

Chemistry, 22.06.2019 21:20, jordan2875
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
You know the right answer?
Pure nitrogen (n2) and pure hydrogen (h2) are fed to a mixer. the product stream has 40.0% mole nitr...
Questions in other subjects:


Mathematics, 10.11.2021 14:00


Mathematics, 10.11.2021 14:00

English, 10.11.2021 14:00

Mathematics, 10.11.2021 14:00


Biology, 10.11.2021 14:00

Physics, 10.11.2021 14:00

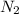 in feed = 40 mole%
in feed = 40 mole%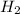 in feed = (100 - 40)% = 60%
in feed = (100 - 40)% = 60%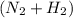 in feed stream.
in feed stream.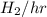 (2 g/mol of
(2 g/mol of 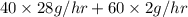
 (as 1 kg = 1000 g)
(as 1 kg = 1000 g)