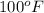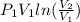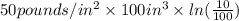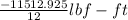Chemistry, 25.09.2019 01:10 saifallahassefa
Avapor is compressed in a frictionless piston-cylinder such that the volume changes from 100 to 10 in3 . the initial pressure is 50 psia and temperature is held constant at 100°f. calculate the work in ft lbf. the vapor can be assumed to be an ideal gas.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:00, AIhunter2884
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

You know the right answer?
Avapor is compressed in a frictionless piston-cylinder such that the volume changes from 100 to 10 i...
Questions in other subjects:

Chemistry, 04.05.2020 23:11

Mathematics, 04.05.2020 23:11


Mathematics, 04.05.2020 23:11

Chemistry, 04.05.2020 23:11



Mathematics, 04.05.2020 23:11

Mathematics, 04.05.2020 23:12

Mathematics, 04.05.2020 23:12

 ) = 100
) = 100 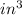
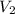 ) = 10
) = 10 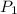 ) = 50 psia = 50
) = 50 psia = 50