
Agas mixture of 20 moles nitrogen and 70 moles hydrogen is fed to a reactor in which ammonia is produced. assuming that the reaction proceeds to completion, determine the composition of the exit gas stream both in mole and mass %. if 75% of nitrogen gets converted, what will be the corresponding fractions?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, anonymous176
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 3

Chemistry, 22.06.2019 09:20, payshencec21
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 14:30, lorrainelopez
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 18:10, sangamlama
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
Agas mixture of 20 moles nitrogen and 70 moles hydrogen is fed to a reactor in which ammonia is prod...
Questions in other subjects:


Mathematics, 05.03.2021 05:50




Mathematics, 05.03.2021 05:50

Mathematics, 05.03.2021 05:50


Mathematics, 05.03.2021 05:50

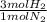 = 60 moles of H₂(g)
= 60 moles of H₂(g) 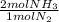 = 40 moles of NH₃(g)
= 40 moles of NH₃(g)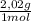 = 20,2 g of H₂
= 20,2 g of H₂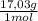 = 681,2 g of NH₃
= 681,2 g of NH₃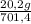 ₓ 100 = 2,9% H₂;
ₓ 100 = 2,9% H₂; 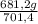 ₓ100 = 97,1% NH₃
ₓ100 = 97,1% NH₃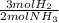 = 45 moles of H₂(g)
= 45 moles of H₂(g) 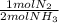 = 15 moles of N₂(g)
= 15 moles of N₂(g) 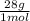 = 140 g of N₂
= 140 g of N₂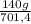 ₓ 100 =20% N₂;
ₓ 100 =20% N₂; 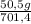 ₓ 100 = 7,2% H₂;
ₓ 100 = 7,2% H₂; 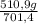 ₓ100 = 72,8% NH₃
ₓ100 = 72,8% NH₃

