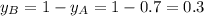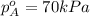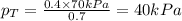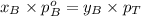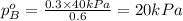Components a and b form ideal solution. at 350 k, a liquid mixture containing 40% (mole) a is in equilibrium with a vapour containing 70% (mole) a. if the vapour pressure of a at 350 k is 70 kpa, what is the vapour pressure of b? (b) 20 kpa (d) 12 kpa (а) 25 kpa (c) 40 kpa

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 07:40, caleb19moody
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2
You know the right answer?
Components a and b form ideal solution. at 350 k, a liquid mixture containing 40% (mole) a is in equ...
Questions in other subjects:

Mathematics, 22.06.2021 09:40

Mathematics, 22.06.2021 09:40

Chemistry, 22.06.2021 09:40


Mathematics, 22.06.2021 09:40





Mathematics, 22.06.2021 09:40

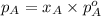 .............(1)
.............(1)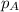 = partial vapor pressure of A
= partial vapor pressure of A = vapor pressure of pure substance A
= vapor pressure of pure substance A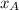 = mole fraction of A
= mole fraction of A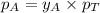 .............(2)
.............(2)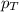 = total pressure of the mixture
= total pressure of the mixture = mole fraction of A
= mole fraction of A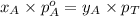
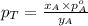 ............(3)
............(3)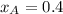 and
and 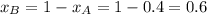
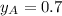 and
and