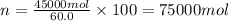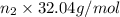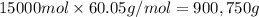Amixture is 20.00 mole% methyl alcohol, 60.0 mole% methyl acetate, and 20.0 mole% acetic acid.
...


Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:50, kyleighmarie05
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 14:00, IdkHowToDoMath
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 20:10, jakhunter354
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for agcl(s) is -916 kj/mol and the hydration enthalpy (dissolution of gaseous ions into water) is -850 kj/mol. how much heat (in joules) is involved in forming 1l of saturated agcl solution (1.8 × 10-4 g / 100 ml water) by dissolving agcl(s)? assume solution volume does not change much upon dissolution. the equations are given below. ag+(g) + cl−(g) æ agcl(s)
Answers: 3
You know the right answer?
Questions in other subjects:


World Languages, 29.09.2019 20:50

History, 29.09.2019 20:50



French, 29.09.2019 20:50


Mathematics, 29.09.2019 20:50

Health, 29.09.2019 20:50

Chemistry, 29.09.2019 20:50

 =45.0 kmol= 45000 mol
=45.0 kmol= 45000 mol