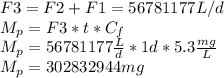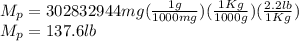Chemistry, 25.09.2019 00:20 deepunalli300p3ur3i
Five million gallons per day (mgd) of wastewater, with a concentration of 10.0 mg/l of a conservative pollutant, is released into a stream having an upstream flow of 10 mgd and pollutant concentration of 3.0 mg/l. (a) what is the concentration in ppm just downstream? (b) how many pounds of substance per day pass a given spot downstream? (you may want the conversions 3.785 l/gal and 2.2 kg/lbm from appendix a.)

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:10, sophiaa23
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 22.06.2019 22:20, icantspeakengles
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 22.06.2019 22:50, kanerobertrosss2213
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
Five million gallons per day (mgd) of wastewater, with a concentration of 10.0 mg/l of a conservativ...
Questions in other subjects:

Mathematics, 25.08.2019 07:30

Mathematics, 25.08.2019 07:30


Mathematics, 25.08.2019 07:30


English, 25.08.2019 07:30

Biology, 25.08.2019 07:30


Social Studies, 25.08.2019 07:30

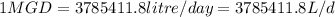
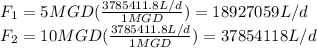
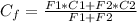
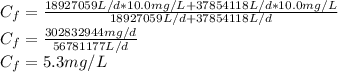
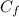 . It is required to calculate per day, let's take a time of t = 1 day.
. It is required to calculate per day, let's take a time of t = 1 day.