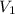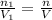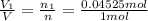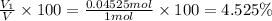Chemistry, 24.09.2019 23:30 zanaplen27
Deep sea divers use a mixture of helium and oxygen to breathe. assume that a diver is going to a depth of 150 feet where the total pressure is 4.42 atm. the partial pressure of oxygen at this depth is to be maintained at 0.20 atm, the same as at sea level. what must be the percent by volume of oxygen in the gas mixture?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:40, cheesecake1919
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 11:00, jodonw5616
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

Chemistry, 22.06.2019 18:50, christhegreat1
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 22:00, cooljariel11
Give more examples of this type of heat transfer:
Answers: 1
You know the right answer?
Deep sea divers use a mixture of helium and oxygen to breathe. assume that a diver is going to a dep...
Questions in other subjects:



Biology, 10.07.2019 23:20


Social Studies, 10.07.2019 23:20


Mathematics, 10.07.2019 23:20

Health, 10.07.2019 23:30


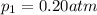
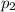
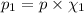 (Dalton law of partial pressure)
(Dalton law of partial pressure)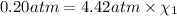
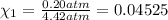
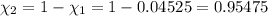
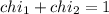
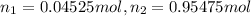
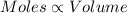 (At temperature and pressure)
(At temperature and pressure)