
Chemistry, 24.09.2019 23:20 Marcynandrew
Amixture of methane and air is capable of being ignited only if the mole percent of methane is between 5% and 15%. a mixture containing 9.0 mole% methane in air flowing at a rate of 700 kg/h is to be diluted with pure air to reduce the methane concentration to the lower flammability limit. calculate the required flow rate of air in mol/h and the percent by mass of oxygen in the product gas. (note: air may be taken to consist of 21 mole% o and 79% n2 and to have an average molecular weight of 29.0.)
(a)n = 20,200 mol air/h; 0.225 kg o2/kg

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, girly37
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 14:20, montanolumpuy
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d. lytic
Answers: 1

Chemistry, 22.06.2019 19:30, gracieisweird12
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 22.06.2019 22:40, destineysarah
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
You know the right answer?
Amixture of methane and air is capable of being ignited only if the mole percent of methane is betwe...
Questions in other subjects:

Mathematics, 23.09.2020 17:01



English, 23.09.2020 17:01

Biology, 23.09.2020 17:01




History, 23.09.2020 17:01

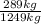 =0,231 kg O₂/ kg air
=0,231 kg O₂/ kg air

