Chemistry, 24.09.2019 23:20 maskythegamer
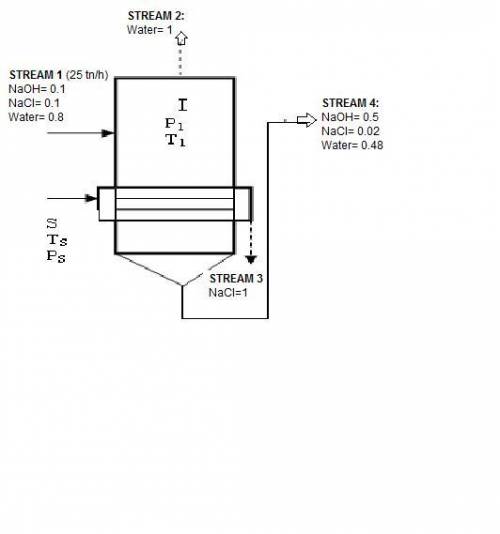
In an evaporator 25 ton / h of a solution of 10% naoh, 10% nacl, and 80% water by weight. during evaporation, the water evaporates and the salt precipitates like crystals they are allowed to settle and are removed. the outgoing concentrated solution of the evaporator contains 50% naoh, 2% nacl and 48% water. based on this information is requested:
1. draw the process flow diagram, indicating each of its streams and compositions (known and unknown).
2. calculate the kilograms of precipitated salt and the kilograms of solution concentrated for every hour of work.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, taralynnn8870
Fugu, also known as puffer fish, is a sushi delicacy that can also be lethal. puffer fish contain a powerful toxin that can kill an adult a few hours after ingestion. sushi chefs who prepare fugu must be specially trained because any contamination of the toxin-free areas of the fish can be deadly. recently this toxin has been put to good use, as scientists have discovered that a purified form of it can treat severe pain in cancer patients. this recent scientific discovery would fall under which area of chemistry? applied biochemistry pure organic chemistry pure physical chemistry applied inorganic chemistry
Answers: 1

Chemistry, 22.06.2019 17:00, emma3216
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 18:50, christhegreat1
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 20:00, aksambo4707
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1
You know the right answer?
In an evaporator 25 ton / h of a solution of 10% naoh, 10% nacl, and 80% water by weight. during eva...
Questions in other subjects:

History, 22.04.2021 21:20

Mathematics, 22.04.2021 21:20


Mathematics, 22.04.2021 21:20


Mathematics, 22.04.2021 21:20

Advanced Placement (AP), 22.04.2021 21:20

SAT, 22.04.2021 21:20