
Chemistry, 24.09.2019 23:11 celibe9391
Aluminum chloride, aich is an inexpensive reagent used in many industrial processes. it is made by treating scrap aluminum with chlorine according to the following equation. 2 aln + 3 cla) → 2 alcl3() a) if 13,49 g of al and 35.45 g of cl2 are allowed to react, how much alcl is produced? b) how many grams of the excess reactant is left? !

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, tamikagoss22
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 04:40, khan2491
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 07:00, vivianni0727p1y30v
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2

Chemistry, 22.06.2019 08:40, jeffcarpenter
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1
You know the right answer?
Aluminum chloride, aich is an inexpensive reagent used in many industrial processes. it is made by t...
Questions in other subjects:




Mathematics, 26.06.2019 00:20

Mathematics, 26.06.2019 00:20


History, 26.06.2019 00:20



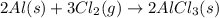
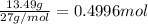
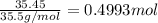
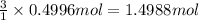 of chlorine gas
of chlorine gas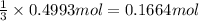 of aluminum
of aluminum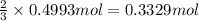 of aluminium chloride .
of aluminium chloride .

