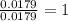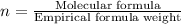Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, cynthiagutierrez65
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1



Chemistry, 22.06.2019 16:00, julesperez22
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
Gallium oxide, ga. oy, forms when gallium is combined with oxygen. a 1.25 g of ga is allowed to reac...
Questions in other subjects:

Mathematics, 06.02.2021 15:00

Social Studies, 06.02.2021 15:00

Mathematics, 06.02.2021 15:00

Mathematics, 06.02.2021 15:00

Social Studies, 06.02.2021 15:00

Physics, 06.02.2021 15:00

Mathematics, 06.02.2021 15:00

Mathematics, 06.02.2021 15:10


Mathematics, 06.02.2021 15:10