
Chemistry, 24.09.2019 22:00 MrTeriffic
Calculate the ph of a water at 25°c that contains 0.6580 mg/l carbonic acid. assume that [h+ ]=[hco3 − ] at equilibrium and neglect the dissociation of water.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, yolo123321
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

Chemistry, 22.06.2019 06:30, dimondqueen511
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 12:50, martinez6221
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1
You know the right answer?
Calculate the ph of a water at 25°c that contains 0.6580 mg/l carbonic acid. assume that [h+ ]=[hco3...
Questions in other subjects:

Mathematics, 20.02.2021 01:00

Mathematics, 20.02.2021 01:00

Mathematics, 20.02.2021 01:00

Mathematics, 20.02.2021 01:00

Mathematics, 20.02.2021 01:00

Mathematics, 20.02.2021 01:00

Business, 20.02.2021 01:00


World Languages, 20.02.2021 01:00

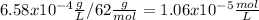
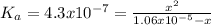
![4.3x10^{-7}.[1.06x10^{-5}-x] = x^{2}\\4.6x10^{-12} - 4.3x10^{-7}x - x^{2} = 0\\x1 = 1.9x10^{-6} \\x2=-2.4x10^{-6}](/tpl/images/0259/3613/28b48.png)
![pH = - Log [H^{+} ] = -Log (1.9x10^{-6}) = 5.7](/tpl/images/0259/3613/91f66.png)



