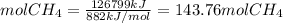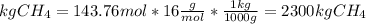The reaction is as follows: ch4 + 202 + co2 + 2h2o the enthalpy of reaction between methane ( 2 ) and oxygen ( 2 ) is exothermic (-882 kj/mol) with respect to the dissipation of methane. it is of interest to generate 126799 kj of energy from the combustion. how many kg of methane will need to be combusted? molecular weight: c-12 kg/kmol h-1 kg/kmol 0 - 16 kg/kmol select one: o a. 2.300 o b. 2300.209 o c. 1.725 o d. 0.009

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, leslyrivera11
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 04:00, amandasantiago2001
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 09:00, SilverTheAmarok
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2

Chemistry, 22.06.2019 18:00, faithabossard
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3
You know the right answer?
The reaction is as follows: ch4 + 202 + co2 + 2h2o the enthalpy of reaction between methane ( 2 ) a...
Questions in other subjects:


Mathematics, 05.01.2021 05:50


Mathematics, 05.01.2021 05:50


Chemistry, 05.01.2021 05:50

History, 05.01.2021 05:50

Mathematics, 05.01.2021 05:50

Mathematics, 05.01.2021 05:50