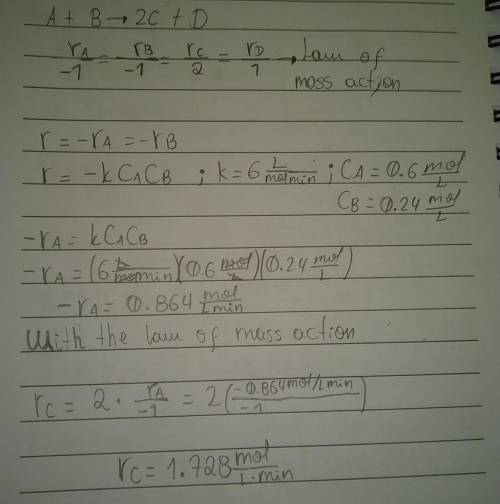Chemistry, 24.09.2019 20:00 soulspiritsa
What is the instantaneous rate of formation of product c given the following information: a. stoichiometric equation a+ b2c+ d b. applicable rate equation is r.-k"ca"cb c. the rate constant is 6.0 liters/(mole-minute) d. the current concentrations of a and b species are ca 0.6 moles/liter and ca 0.24 moles/liter

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, rileyallen4186pd5tgy
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1

Chemistry, 22.06.2019 06:30, yolo123321
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2


Chemistry, 23.06.2019 09:00, AdoNice
A2-kg bowling ball is 1 meter off the ground on a post when it falls. just before it reaches the ground, its traveling 4.4 m/s. assuming that there is no air resistant, which statement is true a. the initial potential energy is less then the final kinetic energy b. the mechanical energy is not conserved c. the mechanical energy is conserved d. the initial potential energy is greater than the final kinetic energy
Answers: 3
You know the right answer?
What is the instantaneous rate of formation of product c given the following information: a. stoich...
Questions in other subjects:





Mathematics, 08.04.2021 09:10


Mathematics, 08.04.2021 09:10

Computers and Technology, 08.04.2021 09:10