
Chemistry, 24.09.2019 03:20 haydencheramie
The combustion of fuel in your car engine requires oxygen gas, which is supplied as air (21% oxygen molecules) into the engine. consider a car that is using 100% ethanol, c2h5oh, as fuel. if your engine intakes 4.73 l of air per minute at 1.00 atm and 25ºc, what is the maximum volume of ethanol (0.789 g/ml) that can be burned per minute? hint: you can ignore the "per minute" information because both the ethanol and air are being quantified per minute. enter your answer to three significant figures in units of ml.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, debordc17
Two friends at different locations want to communicate with each other by sending low energy signals. which of the following methods can they use to communicate? a) produce x-rays using colliding electrons and send them to radios, which capture sound b) send messages using infrared radiation, which travel in the form of waves c) send radio waves through intervening media like radio and television d) produce sound waves using microwaves from heated objects
Answers: 2


Chemistry, 22.06.2019 09:00, phebusadrian01
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1
You know the right answer?
The combustion of fuel in your car engine requires oxygen gas, which is supplied as air (21% oxygen...
Questions in other subjects:




English, 06.02.2021 06:50

History, 06.02.2021 06:50

Chemistry, 06.02.2021 06:50


Mathematics, 06.02.2021 06:50

Health, 06.02.2021 06:50

Law, 06.02.2021 06:50

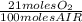 = 0,0406 moles O₂
= 0,0406 moles O₂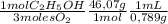 = 0,895 mL of ethanol per minute
= 0,895 mL of ethanol per minute

