
Chemistry, 24.09.2019 01:20 RickandMorty420710
Agas of potassium chlorate molecules kclo3 all decompose into potassium chloride, kcl, and diatomic oxygen, o2. the products and reactants are in a closed container and can all be treated as ideal gases. a. fill in the smallest possible integers that allows the stoichiometry of the reaction equation to be correct: __ kclo3 → kcl o2b. if there are n molecules of potassium chlorate in the initial state, how many product molecules are there

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, carsonjohnsonn
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1

Chemistry, 22.06.2019 08:30, MacenParisi
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2


Chemistry, 22.06.2019 20:30, demarcuswiseman
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
You know the right answer?
Agas of potassium chlorate molecules kclo3 all decompose into potassium chloride, kcl, and diatomic...
Questions in other subjects:


Mathematics, 20.01.2021 14:00

Mathematics, 20.01.2021 14:00

Mathematics, 20.01.2021 14:00






Mathematics, 20.01.2021 14:00

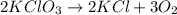
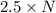 molecules of product.
molecules of product. of particles.
of particles.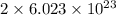 molecules of reactant give
molecules of reactant give 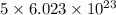 molecules of product
molecules of product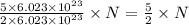 molecules of product.
molecules of product.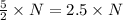 molecules of product are there.
molecules of product are there.

