Chemistry, 23.09.2019 21:10 sparky1234
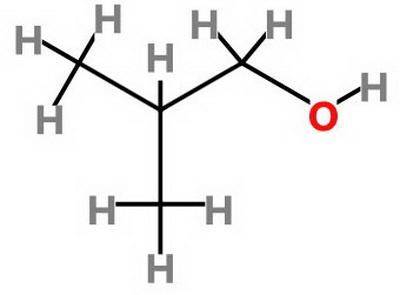
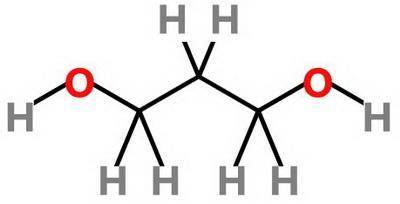
Which statement about 2‑methyl‑1‑propanol, (ch3)2chch2oh , and 1,3‑propanediol, hoch2ch2ch2oh is true? 2‑methyl‑1‑propanol is more soluble in water than 1,3‑propanediol because 2‑methyl‑1‑propanol has a smaller molecular mass. 2‑methyl‑1‑propanol is more soluble in water than 1,3‑propanediol because 2‑methyl‑1‑propanol forms fewer hydrogen bonds with water. 1,3‑propanediol is more soluble in water than 2‑methyl‑1‑propanol because 1,3‑propanediol has a smaller molecular mass. 1,3‑propanediol is more soluble in water than 2‑methyl‑1‑propanol because 1,3‑propanediol can form multiple hydrogen bonds with water.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, hemolelekeakua
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 16:00, graciewyatt6833
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2
You know the right answer?
Which statement about 2‑methyl‑1‑propanol, (ch3)2chch2oh , and 1,3‑propanediol, hoch2ch2ch2oh is tru...
Questions in other subjects:

Mathematics, 19.10.2021 02:50


Health, 19.10.2021 02:50


History, 19.10.2021 02:50

Mathematics, 19.10.2021 02:50