
Chemistry, 22.09.2019 03:20 shealynh52
Considering the limiting reactant, what is the mass of iron produced from 80.0 g of iron(ii)oxide (71.55 g/mol) and 20.0 g of magnesium metal? feof)+ mg() fe)mgo6) a) 62

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, kathleensumter4913
219 grams of iron (iii) oxide reacts with excess carbon according to the reaction equation shown below. fe2o3 + c → fe + co2 after a scientist performs the chemical reaction they find the actual yield of iron to be 57.4 grams. calculate the percent yield of this chemical reaction.
Answers: 1


Chemistry, 22.06.2019 08:00, flakko1899
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1
You know the right answer?
Considering the limiting reactant, what is the mass of iron produced from 80.0 g of iron(ii)oxide (7...
Questions in other subjects:

Mathematics, 13.07.2019 01:00



History, 13.07.2019 01:00



Mathematics, 13.07.2019 01:00

Mathematics, 13.07.2019 01:00

English, 13.07.2019 01:00

Mathematics, 13.07.2019 01:00

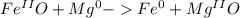
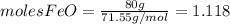 and
and 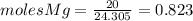 the molecular weight of Mg (24.305) can be readed in the periodic table of elements.so we divide the moles by stoichiometry number (number in front of each compound in the equation) in this case is 1 for both reactants (that is we need 1 mol of FeO and 1 mol of Mg to produce 1 mol of Fe).The lower number obtained was 0.823 for Mg, so Mg is the limiting reactant.
the molecular weight of Mg (24.305) can be readed in the periodic table of elements.so we divide the moles by stoichiometry number (number in front of each compound in the equation) in this case is 1 for both reactants (that is we need 1 mol of FeO and 1 mol of Mg to produce 1 mol of Fe).The lower number obtained was 0.823 for Mg, so Mg is the limiting reactant.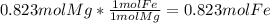 ). To convert from mol of Fe to grams of Fe we would multiply by the molecular weight of Fe
). To convert from mol of Fe to grams of Fe we would multiply by the molecular weight of Fe 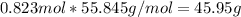 (molecular weight of Fe is readed in the periodic table of elements). So it is produced 45.95 g of iron
(molecular weight of Fe is readed in the periodic table of elements). So it is produced 45.95 g of iron

