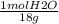Chemistry, 21.09.2019 21:30 floodlife4223
An aqueous solution is listed as being 33.8% solute by mass with a density of 1.15 g/ml, the molar mass of the solute is 145.6 g/mol and the molar mass of water is 18.0 g/mol. a) what is the molality of the solution? b) what is the mole fraction of the solute?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, xojade
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 13:50, awesomegamergurl13
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2
You know the right answer?
An aqueous solution is listed as being 33.8% solute by mass with a density of 1.15 g/ml, the molar m...
Questions in other subjects:



Mathematics, 12.11.2020 20:10


Geography, 12.11.2020 20:10


Chemistry, 12.11.2020 20:10


Mathematics, 12.11.2020 20:10

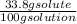 x
x 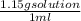 x
x  x
x 
 = 0.232 mol
= 0.232 mol