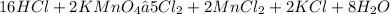Chemistry, 21.09.2019 18:10 noahwhitfield5331
Given the following balanced redox reaction: 2 kmno,+16 hci2kci+2 mncl, +8 h20+5 cl what is the stoichiometric factor relating km o, and mncl, representing the actual numbers of moles of each species that react and are produced according to the equation above?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 02:00, ItzAquaZ1449
Alice did an experiment to find the relationship between the angle at which a ray of light strikes a mirror and the angle at which the mirror reflects the light. she placed a ray box in front of a mirror. she changed the angle at which the light from the ray box struck the mirror and noted the corresponding angle at which the mirror reflected the light. which of the following is the dependent variable in this experiment? the mirror used to reflect the light the ray box used as the source of light angle at which the light from the ray box strikes the mirror angle at which the mirror reflects the light from the ray box
Answers: 2

Chemistry, 23.06.2019 03:00, cabreradesirae4807
Select the correct answer. wax is a nonpolar substance. in which type of substance is it most soluble?
Answers: 2
You know the right answer?
Given the following balanced redox reaction: 2 kmno,+16 hci2kci+2 mncl, +8 h20+5 cl what is the sto...
Questions in other subjects:


Mathematics, 19.09.2019 19:00

Mathematics, 19.09.2019 19:00

Mathematics, 19.09.2019 19:00




Chemistry, 19.09.2019 19:00