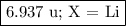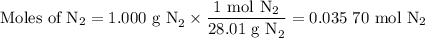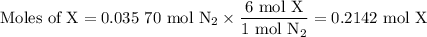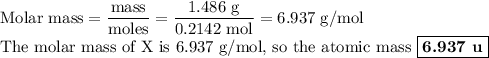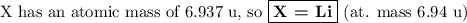Chemistry, 21.09.2019 07:30 MadiAbbott798
Nitrogen reacts with a metal to form a compound in which there are three atoms of the metal for each atom of nitrogen. if 1.486
g of the metal reacts with 1.000 g of nitrogen, what is the calculated atomic mass of the metal?
use your calculated atomic mass to identify the metal. (for your answer, input the proper chemical symbol for element x.)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, isaiahrodriguezsm17
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 18:30, losalobos46
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 22.06.2019 20:00, 20calzoy
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Chemistry, 23.06.2019 00:50, kaseywright3418
Which statement would indicate the presence of an acid
Answers: 3
You know the right answer?
Nitrogen reacts with a metal to form a compound in which there are three atoms of the metal for each...
Questions in other subjects:








Chemistry, 15.08.2020 23:01