
The elementary reaction no2 (g) → 2no (g) + o2 (g) is second order in no2 and the rate constant at 690 k is 1.27 × 101 m-1s-1. the reaction half-life at this temperature when [no2]0 = 0.45 m is s. the elementary reaction no2 (g) → 2no (g) + o2 (g) is second order in no2 and the rate constant at 690 k is 1.27 × 101 m-1s-1. the reaction half-life at this temperature when [no2]0 = 0.45 m is s. 0.17 18 5.7 0.054 0.079

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, penelopegrace04
What is the ph of a solution with a 1.50 × 10−9 m hydroxide ion concentration?
Answers: 3

Chemistry, 21.06.2019 17:10, bettybales1986
What effect does nuclear radiation have on atoms?
Answers: 1

Chemistry, 23.06.2019 01:30, Sonicawesomeness
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2

You know the right answer?
The elementary reaction no2 (g) → 2no (g) + o2 (g) is second order in no2 and the rate constant at 6...
Questions in other subjects:




Mathematics, 11.12.2020 05:50



Mathematics, 11.12.2020 05:50

Social Studies, 11.12.2020 05:50


Mathematics, 11.12.2020 05:50

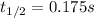
![\frac{1}{[NO_2]}=kt+ \frac{1}{[NO_2]_0}](/tpl/images/0248/0810/446e1.png)
![[NO_2]=\frac{[NO_2]_0}{2} \\](/tpl/images/0248/0810/70bce.png)
![t_{1/2}=\frac{1}{k[NO_2]_0} \\t_{1/2}=\frac{1}{(1.27x10^1\frac{L}{mol*s} )(0.45 \frac{mol}{L} )} \\\\t_{1/2}=0.175s](/tpl/images/0248/0810/f2ea3.png)


