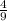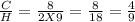Acombustion reaction involves the reaction of a substance with oxygen gas. the complete combustion of any hydrocarbon (binary compound of carbon and hydrogen) produces carbon dioxide and water as the only products. octane is a hydrocarbon that is found in gasoline. complete combustion of octane produces 8 l of carbon dioxide for every 9 l of water vapor (both measured at the same temperature and pressure). what is the ratio of carbon atoms to hydrogen atoms in a molecule of octane?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 21:30, starl0rd211
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 23.06.2019 04:20, vliu732
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1

Chemistry, 23.06.2019 04:31, 24swimdylanoh
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1
You know the right answer?
Acombustion reaction involves the reaction of a substance with oxygen gas. the complete combustion o...
Questions in other subjects:


Mathematics, 25.03.2021 20:00


Mathematics, 25.03.2021 20:00

English, 25.03.2021 20:00



Mathematics, 25.03.2021 20:00

Social Studies, 25.03.2021 20:00