
Chemistry, 20.09.2019 22:00 destinytofell4630
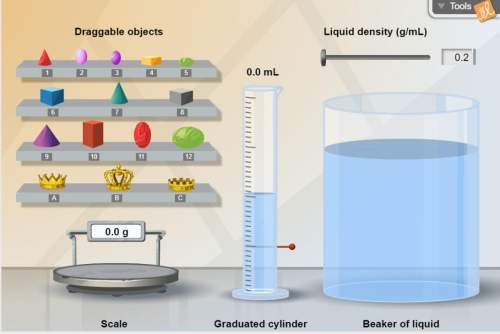
Think about this: how could you devise an easy foolproof way to determine if an object was made of pure gold other than finding the density of the actual object? hint: use the beaker of liquid. briefly explain your method. *

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:00, aksambo4707
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

You know the right answer?
Think about this: how could you devise an easy foolproof way to determine if an object was made of...
Questions in other subjects:

Physics, 09.10.2019 12:20

Mathematics, 09.10.2019 12:20

Chemistry, 09.10.2019 12:20


Social Studies, 09.10.2019 12:20


Health, 09.10.2019 12:20

Biology, 09.10.2019 12:20


History, 09.10.2019 12:20




