
Chemistry, 20.09.2019 17:30 student0724
Akcl solution is prepared by dissolving 40.0 g kcl (molar mass = 74.55 g/mol) in 250.0 g of water (molar mass = 18.01 g/mol) at 25°c. what is the vapor pressure of the solution if the vapor pressure of water at 25°c is 23.76 mm hg?
a) 20.5 mm hg
b) 22.1 mm hg
c) 22.9 mm hg
d) 24.7 mm hg

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, jabper5522
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 04:30, jocelynmarquillo1
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1


Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1
You know the right answer?
Akcl solution is prepared by dissolving 40.0 g kcl (molar mass = 74.55 g/mol) in 250.0 g of water (m...
Questions in other subjects:




Mathematics, 17.04.2020 03:51






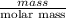
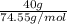
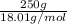
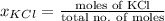
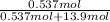
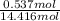
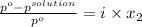
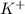 and
and 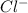 .
.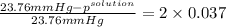
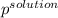 = 22.1 mm Hg
= 22.1 mm Hg


