
Chemistry, 20.09.2019 04:10 brownw2005
the table compares the number of electrons in two unknown neutral atoms.
comparison of electrons
atom number of electrons
a 9
d 11
use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? (5 points)
both are unreactive.
both are highly reactive.
a is unreactive and d is reactive.
a is reactive and d is unreactive.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, Bryanguzman2004
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 14:00, claudia122752
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 23:30, hcllxxhhlpcj
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2
You know the right answer?
the table compares the number of electrons in two unknown neutral atoms.
comparison of electro...
comparison of electro...
Questions in other subjects:

History, 23.05.2020 05:59

English, 23.05.2020 05:59

Physics, 23.05.2020 05:59

Mathematics, 23.05.2020 05:59


Mathematics, 23.05.2020 05:59


English, 23.05.2020 05:59

History, 23.05.2020 05:59

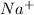 (2, 8)
(2, 8)
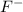 (2, 8)
(2, 8)


