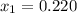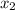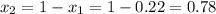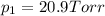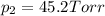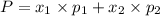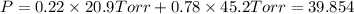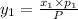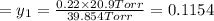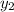Chemistry, 20.09.2019 03:00 aljalloh94
1‑propanol (p∘1=20.9 torr at 25 ∘c) and 2‑propanol (p∘2=45.2 torr at 25 ∘c) form ideal solutions in all proportions. let x1 and x2 represent the mole fractions of 1‑propanol and 2‑propanol in a liquid mixture, respectively, and y1 and y2 represent the mole fractions of each in the vapor phase. for a solution of these liquids with x1=0.220, calculate the composition of the vapor phase at 25 ∘c.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, jeepjose58
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 22:30, xlebrny7831
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 23.06.2019 03:30, rniadsharri16
Select the correct lewis structure for fluorine which is group 7a element?
Answers: 1
You know the right answer?
1‑propanol (p∘1=20.9 torr at 25 ∘c) and 2‑propanol (p∘2=45.2 torr at 25 ∘c) form ideal solutions in...
Questions in other subjects:

English, 15.12.2020 01:10

Biology, 15.12.2020 01:10





Mathematics, 15.12.2020 01:10


Mathematics, 15.12.2020 01:10

Mathematics, 15.12.2020 01:10