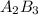When determining the formula for a compound, it is important to know the valence numbers of each element. suppose you want to write a formula for a compound with these two elements: element a = valence 3 element b = valence 2 in the formula, what number will be the subscript for element b?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:50, karlyisaunicorn
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2


You know the right answer?
When determining the formula for a compound, it is important to know the valence numbers of each ele...
Questions in other subjects:


English, 15.01.2021 22:40



Biology, 15.01.2021 22:40

History, 15.01.2021 22:40

Mathematics, 15.01.2021 22:40

Mathematics, 15.01.2021 22:40