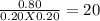Chemistry, 20.09.2019 00:00 corrineikerd
Two different proteins x and y are dissolved in aqueous solution at 37 °c. the proteins bind in a 1: 1 ratio to form xy. a solution that is initially 1.00 mm in each protein is allowed to reach equilibrium. at equilibrium, 0.20 mm of free x and 0.20 mm of free y remain. what is kc for the reaction?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, greekfreekisdbz
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

You know the right answer?
Two different proteins x and y are dissolved in aqueous solution at 37 °c. the proteins bind in a 1:...
Questions in other subjects:

Mathematics, 02.02.2021 02:10

Mathematics, 02.02.2021 02:10


Chemistry, 02.02.2021 02:10


Mathematics, 02.02.2021 02:10

Mathematics, 02.02.2021 02:10



![\frac{[Products]}{[Reactants]}=\frac{[XY]}{[X][Y]}](/tpl/images/0244/5837/0eb39.png)