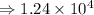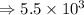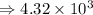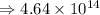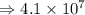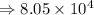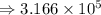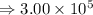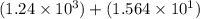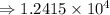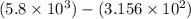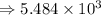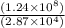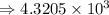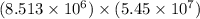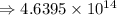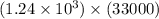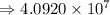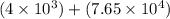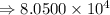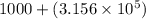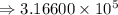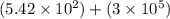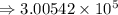Chemistry, 19.09.2019 20:30 ajbrock1004
1) 1.24 x 10^3 + 1.564 x 10^1
2) 5.8 x 10^3 - 3.156 x 10^2
3) 1.24 x 10^8 / 2.87 x 10^4
4) 8.513 x 10^6 x 5.45 x 10^7
5) 1.24 x 10^3 x 33000
6) 4 x 10^3 + 7.65 x 10^4
7) 1000 + 3.156 x 10^5
8) 5.42 x 10^2 + 3 x 10^5

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, alaynagrace1111
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 22:30, brianna5626
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1

Chemistry, 22.06.2019 22:40, lindseyklewis1p56uvi
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization. a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution. part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
1) 1.24 x 10^3 + 1.564 x 10^1
2) 5.8 x 10^3 - 3.156 x 10^2
3) 1.24 x 10^8 / 2.87 x 10^4<...
2) 5.8 x 10^3 - 3.156 x 10^2
3) 1.24 x 10^8 / 2.87 x 10^4<...
Questions in other subjects:

History, 15.01.2020 12:31



Mathematics, 15.01.2020 12:31

Biology, 15.01.2020 12:31




Chemistry, 15.01.2020 13:31