
Chemistry, 19.09.2019 20:30 lulabelles7750
Given the following balanced equation at 120°c: a(g) + b(g) ⇋ 2 c(g) + d(s)(a) at equilibrium a 4.0 liter container was found to contain 1.60 moles of a, and 0.40 moles of b, and 0.40 moles of c, and 1.60 moles of d. calculate kc.(b) if 0.20 moles of b and 0.20 mole of c are added to this system, what will be the new equilibrium concentration of a be? (c) if the volume of the container in which the system is at equilibrium [part (a)] is suddenly halved, what will be the new equilibrium concentrations?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:30, jessixa897192
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

Chemistry, 22.06.2019 19:30, liyahlanderson2232
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 22.06.2019 20:00, batoolishak7475
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
Given the following balanced equation at 120°c: a(g) + b(g) ⇋ 2 c(g) + d(s)(a) at equilibrium a 4.0...
Questions in other subjects:

History, 08.07.2019 12:20

History, 08.07.2019 12:20


Computers and Technology, 08.07.2019 12:20

Social Studies, 08.07.2019 12:20



Mathematics, 08.07.2019 12:30


Mathematics, 08.07.2019 12:30

![\frac{[C]^2}{[A][B]}](/tpl/images/0244/0592/0c73c.png)
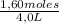 = 0,4 M
= 0,4 M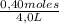 = 0,1 M
= 0,1 M![\frac{[0,1]^2}{[0,4][0,1]}](/tpl/images/0244/0592/6198c.png) = 0,25
= 0,25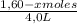

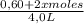
![\frac{[0,60+2x]^2}{[1,60-x][0,60-x]}](/tpl/images/0244/0592/06e92.png)
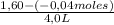 = 0,41 M
= 0,41 M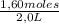 = 0,8 M
= 0,8 M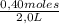 = 0,2 M
= 0,2 M

