
Polonium- 210 , po210 , decays to lead- 206 , pb206 , by alpha emission according to the equation po84210⟶pb82206+he24 if the half-life, /2 , of po210 is 138.4 days , calculate the mass of pb206 produced from a 561.0 mg sample of polonium(iv) chloride, pocl4 , that is left untouched for 333.8 days . assume that the only polonium isotope present in the sample is the po210 isotope. the isotopic molar masses of po210 is 209.98 g/mol and pb206 is 205.97 g/mol .

Answers: 1


Other questions on the subject: Chemistry



You know the right answer?
Polonium- 210 , po210 , decays to lead- 206 , pb206 , by alpha emission according to the equation po...
Questions in other subjects:

Business, 14.10.2019 21:30

Arts, 14.10.2019 21:30

Mathematics, 14.10.2019 21:30



Biology, 14.10.2019 21:30

History, 14.10.2019 21:30

History, 14.10.2019 21:30

Mathematics, 14.10.2019 21:30

 ⟶
⟶  +
+ 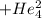
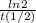
 =0,005008 days^(-1)
=0,005008 days^(-1) = 0,001599 mol of PoCl4
= 0,001599 mol of PoCl4

