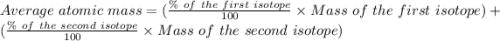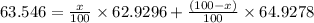Chemistry, 19.09.2019 16:30 xxaurorabluexx
Copper has two naturally occurring isotopes, 63cu (isotopic mass 62.9296 amu) and 65cu (isotopic mass 64.9278 amu). if copper has an atomic mass of 63.546 amu, what is the percent abundance of each isotope? report your answer to 5 significant figures.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, minstcordell4115
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3


Chemistry, 22.06.2019 21:00, alwaysneedhelp84
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
You know the right answer?
Copper has two naturally occurring isotopes, 63cu (isotopic mass 62.9296 amu) and 65cu (isotopic mas...
Questions in other subjects:



History, 20.05.2021 20:00



English, 20.05.2021 20:00

Chemistry, 20.05.2021 20:00

Mathematics, 20.05.2021 20:00

Mathematics, 20.05.2021 20:00