
Chemistry, 19.09.2019 04:30 friendsalwaysbae
Homework 3 write the balanced equations. calculate how many grams of each reactant will be needed to obtain 100.0 grams of the insoluble product formed in the reaction. show complete solutions. 1. aluminum chloride + calcium hydroxide aluminum hydroxide + calcium chloride 2. mercury (ii) oxide mercury + oxygen 3. barium nitrate + copper (ii) sulfate barium sulfate+ copper (ii) nitrate 4. lead (ii) chloride + potassium iodide lead (ii) iodide + potassium chloride 5. sodium sulfide + copper (ii) chloride copper (ii) sulfide + sodium chloride

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 22:30, eduardoguizar8787
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 23.06.2019 01:00, zitterkoph
Which of the following is a physical change? a. burning a piece of wood b. sawing a piece of wood in half c. rust forming on an iron fence d. a copper roof changing color from orange to green
Answers: 1
You know the right answer?
Homework 3 write the balanced equations. calculate how many grams of each reactant will be needed to...
Questions in other subjects:










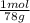 = 1,282 moles of Al(OH)₃.
= 1,282 moles of Al(OH)₃.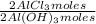 = 1,282 moles of AlCl₃ ×
= 1,282 moles of AlCl₃ × 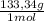 = 170,9 g of AlCl₃
= 170,9 g of AlCl₃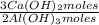 = 1,923 moles of Ca(OH)₂ ×
= 1,923 moles of Ca(OH)₂ × 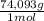 = 142,5 g of Ca(OH)₂
= 142,5 g of Ca(OH)₂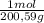 = 0,4985 moles of Hg.
= 0,4985 moles of Hg.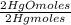 = 0,4985 moles of HgO ×
= 0,4985 moles of HgO × 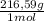 = 108,0 g of HgO
= 108,0 g of HgO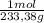 = 0,4285 moles of BaSO₄.
= 0,4285 moles of BaSO₄.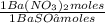 = 0,4285 moles of Ba(NO₃)₂ ×
= 0,4285 moles of Ba(NO₃)₂ × 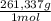 = 112,0 g of Ba(NO₃)₂
= 112,0 g of Ba(NO₃)₂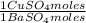 = 0,4285 moles of CuSO₄ ×
= 0,4285 moles of CuSO₄ × 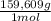 = 68,39 g of CuSO₄
= 68,39 g of CuSO₄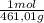 = 0,2169 moles of PbI₂.
= 0,2169 moles of PbI₂.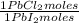 = 0,2169 moles of PbCl₂ ×
= 0,2169 moles of PbCl₂ × 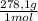 = 60,32 g of PbCl₂
= 60,32 g of PbCl₂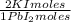 = 0,4338 moles of KI ×
= 0,4338 moles of KI × 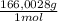 = 72,01 g of KI
= 72,01 g of KI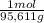 = 1,046 moles of CuS.
= 1,046 moles of CuS.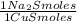 = 1,046 moles of Na₂S ×
= 1,046 moles of Na₂S × 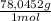 = 81,63 g of Na₂S
= 81,63 g of Na₂S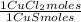 = 1,046 moles of CuCl₂ ×
= 1,046 moles of CuCl₂ × 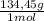 = 140,6 g of CuCl₂
= 140,6 g of CuCl₂


