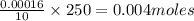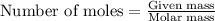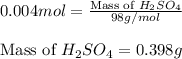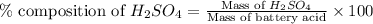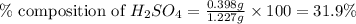Asample of battery acid is to be analyzed for its sulfuric acid content. a 1.00-ml sample weighs 1.227 g . this 1.00-ml sample is diluted to 250.0 ml, and 10.00 ml of this diluted acid requires 35.05 ml of 4.462×10−3 m ba(oh)2 for its titration. what is the mass percent of h2so4 in the battery acid?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, micvar9646
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 06:00, josmanu235
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 21:50, SoccerAllStar2
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

Chemistry, 23.06.2019 11:30, nadine6085859r
Which of the following is a property of nonmetals? a. nonmetals are ductile. b. nonmetals have a shiny luster. c. nonmetals have high density. d. nonmetals are nonconductors.
Answers: 1
You know the right answer?
Asample of battery acid is to be analyzed for its sulfuric acid content. a 1.00-ml sample weighs 1.2...
Questions in other subjects:

Biology, 18.10.2020 14:01


Mathematics, 18.10.2020 14:01

Geography, 18.10.2020 14:01




Social Studies, 18.10.2020 14:01


Mathematics, 18.10.2020 14:01

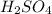 in the battery acid is 31.9 %
in the battery acid is 31.9 %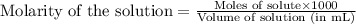
 solution =
solution = 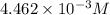
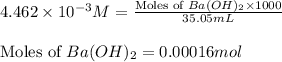
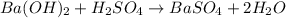
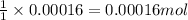 of sulfuric acid.
of sulfuric acid.