
Chemistry, 19.09.2019 01:00 student0724
If a chemist wishes to dilute a 1.000 × 10^3 mg/l stock solution to prepare 5.000 × 10^2 ml of a working standard that has a concentration of 7.000 mg/l, what volume of the 1.000 × 10^3 mg/l standard solution is needed?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:50, rndschopplein
Achemical reaction (also known as a chemical change) produces substances that are chemically different from the starting materials. an example of a chemical reaction is the formation of water from hydrogen and oxygen gas. in a physical change, a substance changes its physical appearance but not its chemical identity. an example of physical change is the formation of liquid water from solid water, a familiar process called melting. physically, liquid water looks very different from solid water (ice) but the chemical identity, water, is the same for both. which of following changes that affect the composition of our atmosphere involve physical changes and which involve chemical reactions? oxygen gas changes to ozone during thunderstorms carbon dioxide is produced by the combustion of gasoline in an automobile engine. when coal, oil, and natural gas are decomposed in landsills they produce methane gas. freezing rain develops when a warm air mass overrides a cold air mass. fog forms from water vapor when the temperature drops below the dew point
Answers: 1

Chemistry, 22.06.2019 09:30, raizagisselle1694
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 21:30, djdjdjdbdbjx
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
If a chemist wishes to dilute a 1.000 × 10^3 mg/l stock solution to prepare 5.000 × 10^2 ml of a wor...
Questions in other subjects:





Physics, 11.05.2021 08:10

Business, 11.05.2021 08:10

Physics, 11.05.2021 08:10



Mathematics, 11.05.2021 08:10

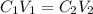
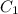 = molarity of stock solution =
= molarity of stock solution = 
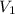 = volume of stock solution = ?
= volume of stock solution = ? = molarity of diluted solution =
= molarity of diluted solution = 
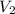 = volume of diluted solution =
= volume of diluted solution =  = 0.5 L (1L=1000 ml)
= 0.5 L (1L=1000 ml)



